Mg Valence Electrons Simple Guide for Students
When studying chemistry, you often hear about mg valence electrons. These electrons are very important. They tell us how elements will react with each other. In this article, you’ll learn everything about it clearly and simply.
What Are Valence Electrons?
Before we learn about mg valence electrons, let’s know what valence electrons really are. Valence electrons are electrons in the outermost energy level of an atom. These electrons help atoms bond or react with other atoms. When atoms meet, valence electrons do all the talking and bonding. Atoms want to become stable by filling their outer shell with electrons. The number of valence electrons tells us how likely an atom is to bond with other atoms. So, valence electrons matter greatly in chemistry.
Basic Facts
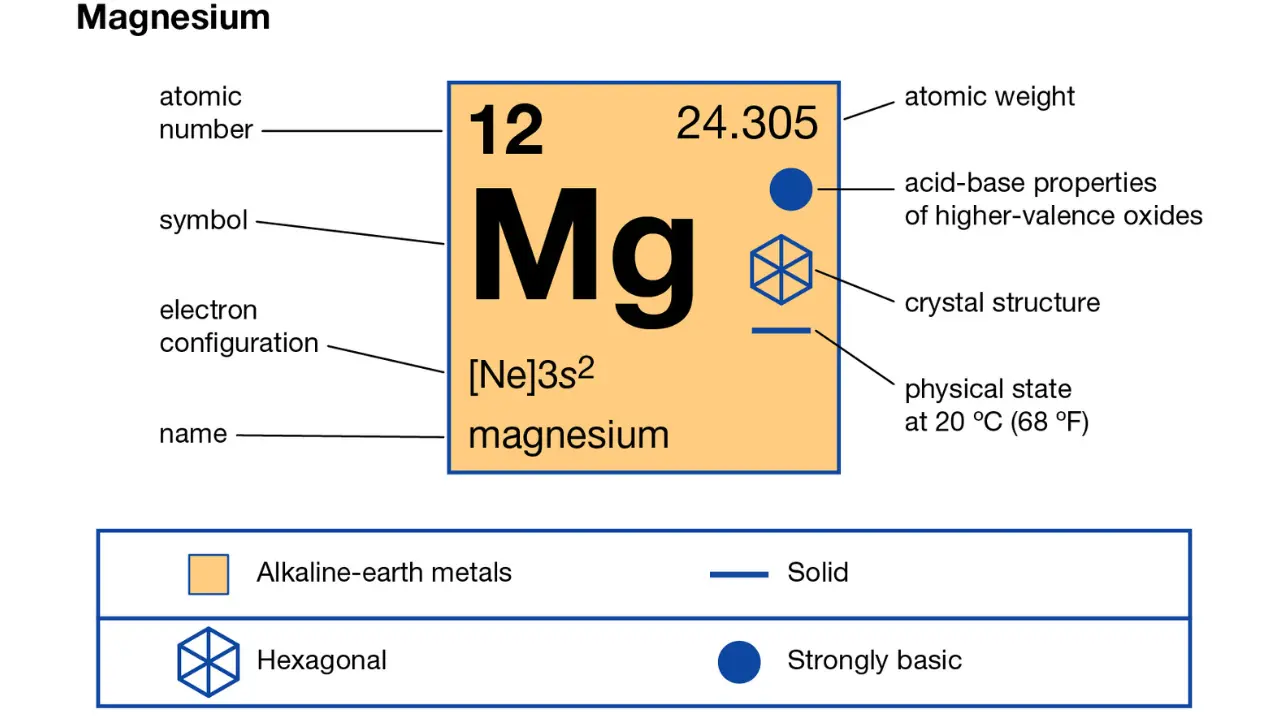
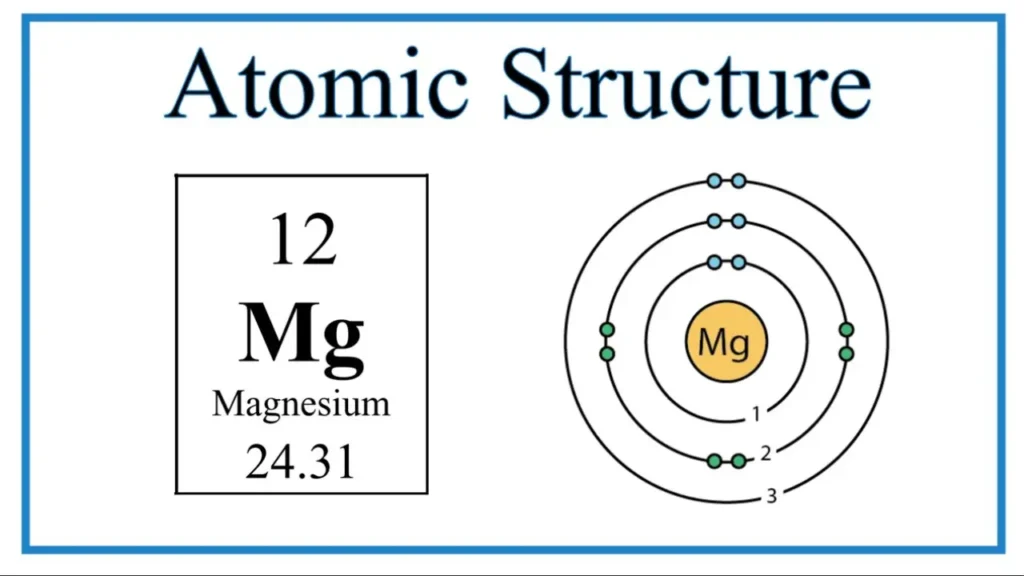
Magnesium, or Mg, is an element with an atomic number of 12. It means magnesium has 12 electrons spinning around its center. But only the outermost electrons are called valence electrons. So, how many mg valence electrons are there? Magnesium has two valence electrons. The electron structure of magnesium is 1s² 2s² 2p⁶ 3s². See that 3s² at the end? It means two electrons exist in the outermost shell. Those two electrons are this.
Why These Are Important
Knowing these helps us predict how magnesium behaves. Since magnesium has two valence electrons, it tends to lose them easily. When magnesium gives away these electrons, it forms a stable ion called Mg²⁺. Magnesium often forms ionic bonds with elements like oxygen or chlorine. Losing two electrons allows magnesium to become stable. So, this explain why magnesium reacts easily with some elements and forms stable compounds.
Affect Chemical Bonds
The two mg valence electrons affect magnesium’s chemical reactions. For example, when magnesium meets oxygen, magnesium gives away its two electrons. Oxygen needs two electrons to become stable. Magnesium gives them away, and oxygen gladly takes them. This exchange forms magnesium oxide (MgO). The reaction makes both atoms stable and happy. So, this play a big role in magnesium oxide formation.
Let’s see this clearly in a simple table:
| Atom | Electrons Needed | Electrons Lost | Ion Formed |
|---|---|---|---|
| Magnesium | 0 | 2 | Mg²⁺ |
| Oxygen | 2 | 0 | O²⁻ |
Examples
You see reactions involving mg valence electrons in daily life. For example, magnesium reacts with chlorine gas. Chlorine needs one electron, but magnesium has two valence electrons. Magnesium solves this problem by giving electrons to two chlorine atoms. The result is magnesium chloride (MgCl₂). Again, it explain clearly why magnesium easily forms compounds.
Influence Stability
Atoms become stable by having full or empty outer electron shells. Magnesium becomes stable by losing two electrons. After losing these electrons, it has a stable shell of eight electrons (2,8). Losing two electrons makes magnesium stable, forming Mg²⁺ ions. Stability is key in chemistry, and it clearly explain magnesium’s stability.
Mg Valence Electrons and the Periodic Table
Magnesium sits in Group 2 of the periodic table. All elements in this group have two valence electrons. Calcium, magnesium, and beryllium behave alike chemically because of their two valence electrons. Understanding it helps you understand many other Group 2 elements. You see patterns clearly thanks to these electrons.
Here’s a table of Group 2 elements and their valence electrons:
| Element | Symbol | Valence Electrons |
|---|---|---|
| Beryllium | Be | 2 |
| Magnesium | Mg | 2 |
| Calcium | Ca | 2 |
| Strontium | Sr | 2 |
| Barium | Ba | 2 |
Real Life Uses
Knowing about mg valence electrons helps scientists use magnesium effectively. Magnesium is in many products we use daily. Cars, airplanes, and bicycles often contain magnesium alloys. These alloys are strong, lightweight, and resist corrosion. Magnesium’s chemical behavior, due to its valence electrons, helps us create safe and strong materials.
Common Misunderstandings
Students sometimes get confused about valence electrons. They think magnesium has 12 valence electrons because it has 12 electrons total. This misunderstanding is very common among learners. Remember, only electrons in the outer shell are valence electrons. Magnesium has only two outer electrons. So, it equal two, not twelve.
Easy Tips to Remember
Students easily forget facts in chemistry. Here’s how you can remember these:
- Think “Group 2 means two electrons”.
- Remember, magnesium wants to lose two electrons to be stable.
- Always link magnesium with two valence electrons in your mind.
Frequently Asked Questions
How many mg valence electrons are there?
Magnesium has 2 valence electrons in its outer shell.
Why are these important?
They help magnesium form chemical bonds by giving away electrons to become stable.
What ion does magnesium form after losing electrons?
Magnesium becomes a stable Mg²⁺ ion after losing its two valence electrons.
Does every element in magnesium’s group have the same valence electrons?
Yes, all elements in Group 2 have 2 valence electrons just like magnesium.
Final Thoughts
Understanding mg valence electrons isn’t hard. These electrons help magnesium react chemically. Magnesium forms strong bonds by giving away two valence electrons easily.
Remember, valence electrons help explain reactions and stability. Magnesium’s two valence electrons are key to its chemical behavior. By learning clearly about these electrons, chemistry becomes simpler and easier to grasp.
In summary, here’s what you’ve learned about it:
- Magnesium has two valence electrons.
- It easily loses these electrons to become stable.
- Losing two electrons makes magnesium form ionic bonds.
Learning about it gives you a solid start in chemistry. Keep this knowledge clear, and chemistry will feel easier and more fun.






